Introduction
Since the comparison between plant-based and animal-inclusive diets can quickly spiral out of control, it’s worth taking a second to assuage tempers on both sides before delving deeper. This is not an ethics article. This is not an article brimming with cherry-picked data to support a certain viewpoint. This is simply an objective discussion on the scientific findings of the difference between plant and animal proteins and how they affect muscle growth. Not potential health markers, not spirituality, not climate change, just muscle growth. All other factors are for arguments far outside the scope of this article. As always, we encourage healthy, evidence-based discussion and greatly appreciate any and all comments. However, if you feel strongly either way about what is presented here, make sure you’re taking the time to pen a clear and concise statement devoid of personal opinion, belief, or poorly-sourced data. No one is here to change anyone’s mind about anything, we just want to talk facts.
Hopefully this preemptive statement does the trick in preventing tempers from flaring, but it is 2019, so I’m sure someone will find a way around it. We can’t worry about that forever, though, so let’s get started.
Why Compare?
It’s worth taking the time to compare protein sources because:
1) Bodybuilding is a very popular activity centered on muscular development and aesthetics (14).
2) Health, longevity, and quality of life are all greatly correlated with muscle mass and strength (2).
3) Muscle mass is predicated on the balance between muscle protein synthesis and protein breakdown (29).
4) Different sources of protein can induce different effects on the rate of muscle protein synthesis (29).
Therefore, comparing animal protein sources with plant protein sources makes perfect sense. Plant and animal proteins can induce different rates of muscle protein synthesis which can affect muscle growth for the bodybuilder and muscle mass in the individual interested in longevity and quality of life. So let’s dig in.
What Drives Muscle Protein Synthesis?
As mentioned above, muscle mass is either increased, maintained, or decreased depending on the balance between protein synthesis and protein breakdown. Positive protein balance elicits increases in muscle size and is driven by an increase in protein synthesis (32,34). Negative protein balance can occur from a lack of food and can ultimately cause a decrease in muscle size due to protein breakdown occurring at a faster rate than protein synthesis.
The two main precursors to protein synthesis are exercise and food intake – primarily protein (13,18,21). Most forms of exercise will cause an increase in muscle protein synthesis with resistance training leading the pack (21). However, damaging exercise (like heavy training) can also increase protein breakdown which necessitates protein intake to drive protein synthesis higher and create a positive protein balance.
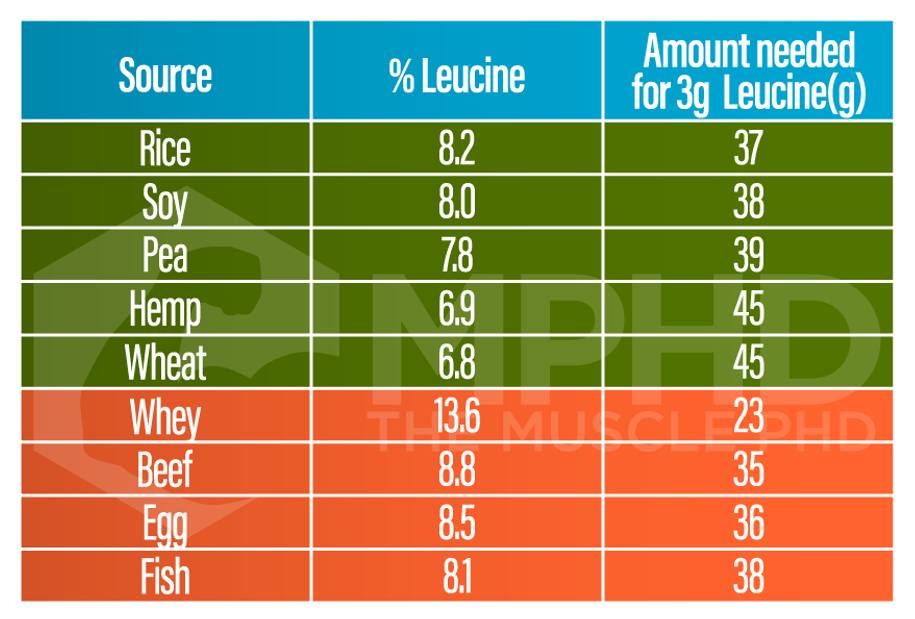
Protein synthesis by way of food is mostly driven by essential amino acids (EAAs). EAAs, and especially BCAAs act as signaling molecules that can help kick start the protein synthesis process (1). Leucine appears to be the most important EAA for stimulating muscle protein synthesis (11,21,28,29) so the majority of the comparisons between protein sources focuses on leucine content. Leucine and EAA content certainly play a role in how well a protein source can stimulate protein synthesis, but other factors are involved as well.
Protein Quality, Kinetics, and Anabolic Potential
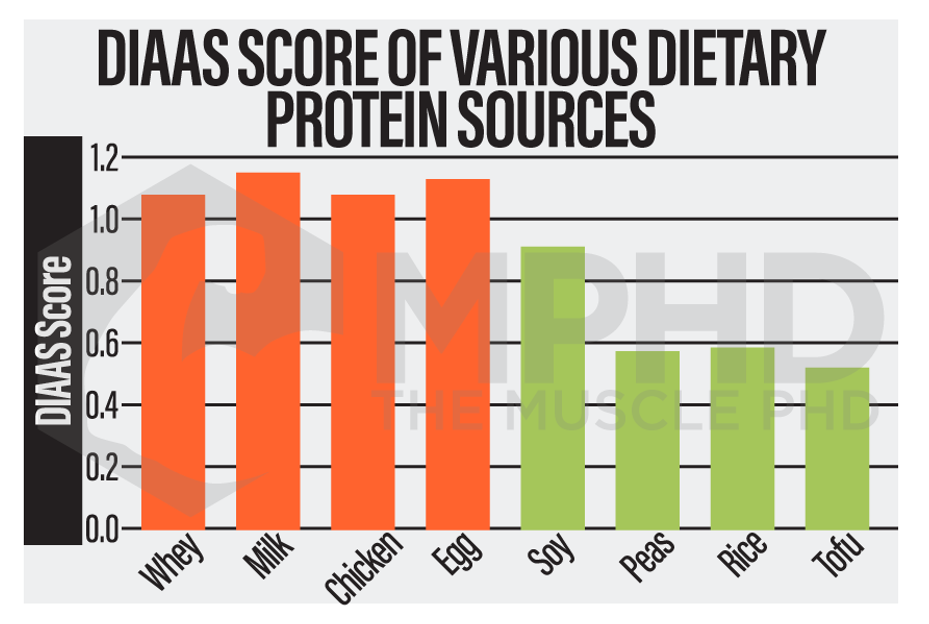 Protein quality is highly related to EAA and leucine content and, up until recently, was measured by the PDCAAS scale, which stands for Protein Digestibility Corrected Amino Acid Score. However, this rating system has started to phase out and is being replaced by the DIAAS, which stands for the Dietary Indispensable Amino Acid Score (15). Both systems are used to measure how much of a certain protein source would be needed to avoid protein deficiency (29). Avoiding deficiency certainly isn’t the same thing as optimizing lean mass gains, so these scores aren’t the most applicable right off the bat. Both systems are great for assessing protein quality and bioavailability, but that doesn’t necessarily correlate with the anabolic potential of a protein source (29). For example, soy and beef protein have similar PDCAAS scores, but beef has been shown to be more effective than soy protein at increasing protein synthesis levels (20,29).
Protein quality is highly related to EAA and leucine content and, up until recently, was measured by the PDCAAS scale, which stands for Protein Digestibility Corrected Amino Acid Score. However, this rating system has started to phase out and is being replaced by the DIAAS, which stands for the Dietary Indispensable Amino Acid Score (15). Both systems are used to measure how much of a certain protein source would be needed to avoid protein deficiency (29). Avoiding deficiency certainly isn’t the same thing as optimizing lean mass gains, so these scores aren’t the most applicable right off the bat. Both systems are great for assessing protein quality and bioavailability, but that doesn’t necessarily correlate with the anabolic potential of a protein source (29). For example, soy and beef protein have similar PDCAAS scores, but beef has been shown to be more effective than soy protein at increasing protein synthesis levels (20,29).
The anabolic potential of a protein source is dependent on several factors, including digestibility, amino acid absorption kinetics (12,19), and EAA composition (26,32) with leucine being the most important (28,29). Therefore, the overall potential of a protein source to encourage muscle growth is dependent on its quality and overall anabolic potential. So how do protein sources compare?
Animal vs Plant Proteins: EAAs, Protein Kinetics, Digestibility, and Quality
From the charts in this section, it’s easy to see that animal-based proteins consistently have higher EAA contents and higher leucine contents (29).
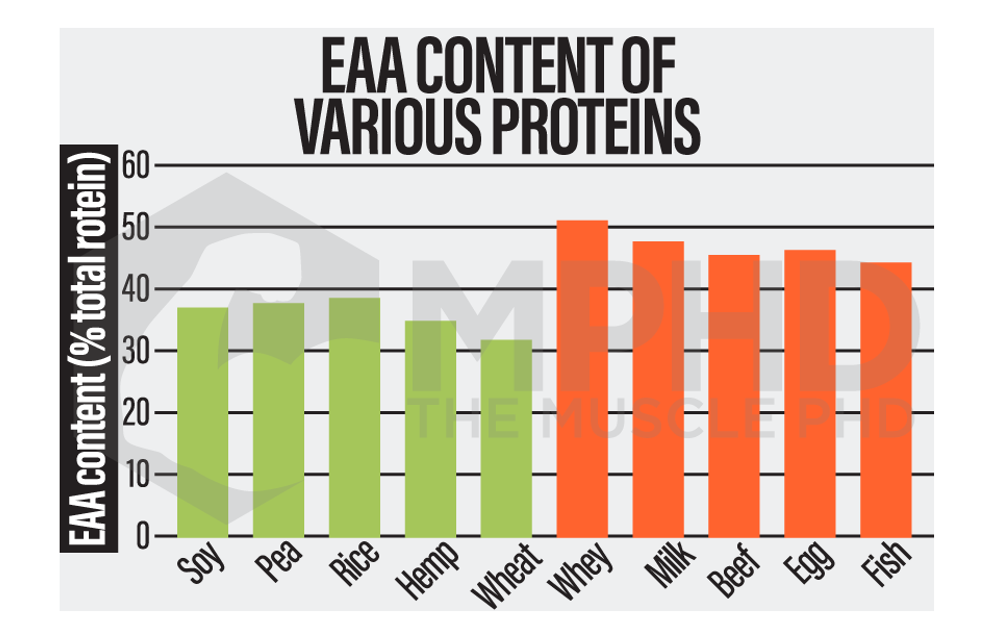 When protein kinetics are considered, research shows that plant proteins actually exhibit lower levels of digestibility when compared to animal proteins (7). This is typically measured by the proportion of amino acids that are digested and absorbed and thus available in a form that can contribute to protein synthesis. Animal proteins typically present with rates over 90% while plant proteins usually range between 45-80% (7). However, isolated forms of plant protein, such as soy isolate or wheat gluten, often score above 90% as they are devoid of things like fiber or phytic acid which may affect digestibility (7,29).
When protein kinetics are considered, research shows that plant proteins actually exhibit lower levels of digestibility when compared to animal proteins (7). This is typically measured by the proportion of amino acids that are digested and absorbed and thus available in a form that can contribute to protein synthesis. Animal proteins typically present with rates over 90% while plant proteins usually range between 45-80% (7). However, isolated forms of plant protein, such as soy isolate or wheat gluten, often score above 90% as they are devoid of things like fiber or phytic acid which may affect digestibility (7,29).
 In addition, amino acids from plant proteins are more likely to be converted to urea than animal proteins (4,5,8,9). This lowers the potential of plant-based proteins to increase protein synthesis as fewer amino acids will be available to promote and contribute to protein synthesis. This is more than likely due to the lack of specific EAAs in many plant proteins. This can lead to an unfavorable amino acid mixture in the gut which increases the amount of amino acids that get shuttled to the liver, ultimately stimulating ureagenesis, or, the production of urea (23). This theory has been somewhat confirmed in studies showing that soy protein causes a much greater increase in amino acid oxidation compared to milk protein when both are consumed in the same amount (35). Overall, fewer amino acids from plant proteins are used for muscle protein synthesis (29).
In addition, amino acids from plant proteins are more likely to be converted to urea than animal proteins (4,5,8,9). This lowers the potential of plant-based proteins to increase protein synthesis as fewer amino acids will be available to promote and contribute to protein synthesis. This is more than likely due to the lack of specific EAAs in many plant proteins. This can lead to an unfavorable amino acid mixture in the gut which increases the amount of amino acids that get shuttled to the liver, ultimately stimulating ureagenesis, or, the production of urea (23). This theory has been somewhat confirmed in studies showing that soy protein causes a much greater increase in amino acid oxidation compared to milk protein when both are consumed in the same amount (35). Overall, fewer amino acids from plant proteins are used for muscle protein synthesis (29).
All of the above can be summarized in the DIAAS comparison between various plant and animal protein sources, shown in the chart in the previous section. Animal proteins consistently score higher than plant proteins (15,22).
Applicable Comparisons
So we now have all of the background science and theories behind how animal and plant proteins might differ, let’s see how this plays out in the real world. In acute ingestion, soy protein does not increase protein synthesis to the same degree as whey protein (24), skim milk (33), or beef (21). Other forms of plant-based proteins don’t have nearly as much literature on their effects on protein synthesis as they’re not as readily available or common as soy (29). Therefore any claims about the gains-effectiveness of a plant-based protein other than soy should be met with appropriate skepticism.
This acute increase in muscle protein synthesis has also been shown to affect gains in the long term. Milk proteins have been shown to be more effective for lean mass gains compared to soy proteins when both are taken in the same amount and same time frame (10,31). In addition, fish eating a plant protein diet compared to a normal fish chow diet actually experienced decreases in muscle fiber size (27). However, studies do show that plant protein can be beneficial for growth, there’s just one caveat: you have to eat more!
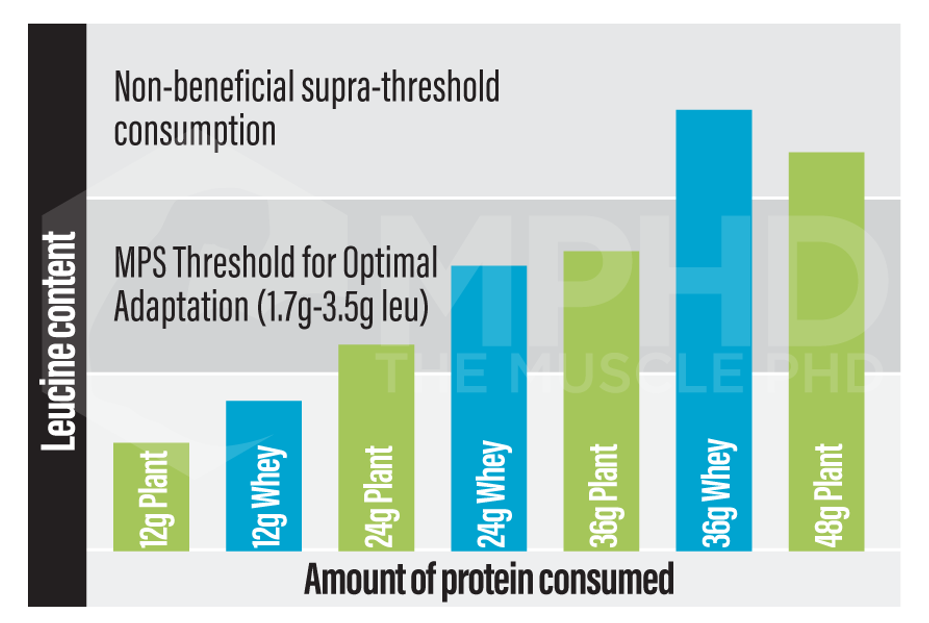 Studies that provided higher amounts of both milk and plant proteins to subjects after a workout saw similar gains in lean mass over the long term between protein sources (6,11). The reason for this is that both studies used at least 30g of plant-based protein. Since a higher amount of plant protein was used, the necessary leucine threshold of 2-3g was met (17,26). If you reference the above chart concerning leucine content and protein sources, it’s easy to see that plants and whey protein could deliver similar amounts of leucine, you just have to take in more plant protein. Since the upper limit of the leucine threshold exists somewhere between 3-4g (11), taking in higher amounts of both will probably lead to similar increases in protein synthesis. Whey will provide leucine beyond the threshold which probably offers no additional benefits, while soy will be right in the wheelhouse of the threshold.
Studies that provided higher amounts of both milk and plant proteins to subjects after a workout saw similar gains in lean mass over the long term between protein sources (6,11). The reason for this is that both studies used at least 30g of plant-based protein. Since a higher amount of plant protein was used, the necessary leucine threshold of 2-3g was met (17,26). If you reference the above chart concerning leucine content and protein sources, it’s easy to see that plants and whey protein could deliver similar amounts of leucine, you just have to take in more plant protein. Since the upper limit of the leucine threshold exists somewhere between 3-4g (11), taking in higher amounts of both will probably lead to similar increases in protein synthesis. Whey will provide leucine beyond the threshold which probably offers no additional benefits, while soy will be right in the wheelhouse of the threshold.
Conclusions
So really the takeaway here is this: animal proteins are more effective for promoting protein synthesis than plant proteins. But! All you have to do to even the playing field is eat greater amounts of plant protein. The above leucine threshold seems necessary for driving muscle protein synthesis, so all one has to do is find the right amount of their desired protein source that lands them in the sweet spot of the leucine threshold. If this step isn’t taken, it’s very easy to under-consume protein during a plant-based diet which can reduce overall lean mass, strength, and quality of life (2).
If one does wish to subscribe to a plant-based diet while attempting to maximize muscle growth, creatine and beta-alanine supplementation should be considered as dietary provisions of both will be much lower (22,30). In addition, vegan/vegetarian diets and the protein sources within their confines contain much higher amounts of antioxidants (30). While antioxidants can improve endurance exercise performance, they can actually impair muscle growth (3).
Quite frankly, a plant-based diet is not optimal for muscle growth. Anyone trying to claim the opposite has some serious soul searching to do. But that doesn’t mean muscle growth is impossible! Take the information cited in this piece and use it to your advantage if you want to design a plant-based diet primed for muscle growth. Plan on eating at least 30g of plant protein at each meal to maximize muscle protein synthesis and try to avoid antioxidants around your workouts as much as possible. Taking digestive enzymes can also help improve the protein kinetics and digestibility of plant proteins (16).
 Again, ethics and morality are far from the contents of this article. If you choose to lead a plant-based lifestyle, I completely respect that. That’s your choice and everyone else’s diet is their choice. However, it’s important to understand the objective and non-biased science behind plant-based diets and muscle growth. Growth can absolutely be achieved on a plant-based diet, however, specific steps must be taken to maximize growth – just like consuming a diet with both animal and plant sources. Optimizing your diet, no matter your primary food source, is a major component of making gains.
Again, ethics and morality are far from the contents of this article. If you choose to lead a plant-based lifestyle, I completely respect that. That’s your choice and everyone else’s diet is their choice. However, it’s important to understand the objective and non-biased science behind plant-based diets and muscle growth. Growth can absolutely be achieved on a plant-based diet, however, specific steps must be taken to maximize growth – just like consuming a diet with both animal and plant sources. Optimizing your diet, no matter your primary food source, is a major component of making gains.
Easter Eggs
If you’re interested in a more in-depth discussion, check out the extensive review by van Vliet, Burd, and van Loon (2015) here. This review was of major assistance while drafting this article so credit is certainly due.
Note: One source in this piece was determined to potentially have a conflict of interest and/or bias (20). However, this source is only used to support a single claim that aligns well with other claims throughout the article.
Update
A recent article (read here) has caused a decent stir in the nutrition community – especially coming from those on the plant-based side. Several plant-based dieters have lauded this paper as the “truth” that the omnivorous community is choosing to ignore. Since the plant-based community seems to be quick to point out shortcomings and conflicts of interest in research that doesn’t support their bias, let’s take a critical review at this piece.
Right off the bat, even the abstract states, “Given the growing shifts in recommendations from nutrition health professionals for people to transition to more plant-based, whole-food diets, additional scientific evidence-based communications confirming the protein adequacy of vegetarian and vegan diets is warranted.” Notice that the authors state that we need data confirming the adequacy of plant-based diets, not examining the adequacy. We haven’t even gotten past the abstract and we’ve already hit a speed bump of bias.
Following this hiccup, the plant-based community has been trying to use this review as evidence supporting the idea that a vegan/vegetarian diet supplies plenty of protein. The article highlights two separate reports covering over 108,000 “meat eaters” and nearly 1600 “vegans.” The average daily protein intake reported by the meat eaters in these questionnaires was 87g/day vs 62g/day reported by vegans. This represents a roughly 40% difference in daily protein intake between the two populations. While this doesn’t quite reach the threshold of the protein spread theory (estimates how much additional protein one would need to make better gains, read here), it’s certainly an interesting difference. Regardless, let’s soldier on.
The point that is most misrepresented or taken out of context in this review is the contention that plant-based diets provide sufficient protein. The authors support this statement by taking the 0.99g/kg/day protein intake reported in one questionnaire and compare this number to the “Estimated Average Requirement” of 0.66g/kg/day of protein and the Recommended Daily Allowance (RDA) of 0.8g/kg/day. So, now begs the question, who is this protein intake sufficient for? Sedentary adults. That’s about it.
This particular review does not even attempt to cover athletes. I’m not sure why, but I have a hunch that delving into sports nutrition wouldn’t exactly support their conclusion mentioned in the paragraph above. The typical recommendation for athletes is 1.4-2.0g/kg/day of protein with strength athletes (like bodybuilders) likely benefiting from even greater intakes (read here). Obviously, no matter your dietary preferences, you’re going to have to increase daily protein intake to support muscle hypertrophy. But the plant-based community has been taking the above conclusion far out of context and is applying it to athletes and bodybuilders.
Moving on.
The article also questions the usefulness of the PDCAAS/DIAAS scale previously discussed earlier on in this piece. While this scale is not perfect, it represents the current working knowledge in the scientific community. I also hope to see improvements to the scale, but I’m skeptical that any improvements would skew the data towards favoring plant proteins due to many of the issues we’ve already discussed above. I look forward to the evolution of this model.
Lastly, the review admits that certain modifications may be required for older adults consuming a plant-based diet. Since older adults typically do not digest and/or absorb protein as well as younger adults, they likely need a greater protein intake to achieve maximal levels of protein synthesis. This would require a greater intake of leucine to help stimulate protein synthesis. The authors simply state that this issue is a, “… subject of interest for future research.”
The discussion on older adults can likely be better compared to athletes and bodybuilders. These populations will also have a need for both greater protein and greater leucine intake. Many studies only examine protein synthesis, rather than overall protein balance. So, we often see protein synthesis maxed out around 25 grams of protein, whether at rest or following exercise (read here). However, this doesn’t account for the increase in protein breakdown that occurs following training. Several types of exercise can induce protein breakdown, so protein intake now has to account for both repair/replacement and addition of muscle proteins. We see evidence of this in studies showing that protein balance can actually be negative following resistance training without protein intake (read here). Therefore, greater protein (and leucine) intake is required to account for both repair/replacement, and addition of new muscle proteins. This is why we see increased recommended daily intakes for athletes and/or bodybuilders.
Oh, and one more thing – the plant based community often seeks to declare conflicts of interest and/or bias when reading studies that don’t support their “side.” Yet, they seem to have glossed over the fact that the primary investigator of this review is the, “… scientific leader of a research contract with Terres Univia, the French Interbranch organization for plant oils and proteins.” Hmmm. Let’s only point out potential conflicts of interest when it supports your bias, huh? This is not saying that we have any reason to believe that this author is up to no good, it’s just an interesting note of hypocrisy.
We understand that this a long-winded review of this piece, but it’s important to keep up with the information surrounding this controversy. This critique also makes it seem like we’re completely against plant-based diets. And that’s not at all the case. We’re just against bad science, misrepresentation of science, and taking data out of context. When the information in this review stays in the context of the population in question, it’s perfectly fine. But trying to apply it to athletic and/or bodybuilding populations is just silly.
References
- Atherton, P. J., Smith, K., Etheridge, T., Rankin, D., & Rennie, M. J. (2010). Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids, 38(5), 1533-1539.
- Aubertin-Leheudre, M., & Adlercreutz, H. (2009). Relationship between animal protein intake and muscle mass index in healthy women. British Journal of Nutrition, 102(12), 1803-1810.
- Bjørnsen, T., Salvesen, S., Berntsen, S., Hetlelid, K. J., Stea, T. H., Lohne‐Seiler, H., … & Haugeberg, G. (2016). Vitamin C and E supplementation blunts increases in total lean body mass in elderly men after strength training. Scandinavian Journal of Medicine & Science in Sports, 26(7), 755-763.
- Bos, C., Juillet, B., Fouillet, H., Turlan, L., Daré, S., Luengo, C., … & Gaudichon, C. (2005). Postprandial metabolic utilization of wheat protein in humans. The American journal of Clinical Nutrition, 81(1), 87-94.
- Bos, C., Metges, C. C., Gaudichon, C., Petzke, K. J., Pueyo, M. E., Morens, C., … & Tome, D. (2003). Postprandial kinetics of dietary amino acids are the main determinant of their metabolism after soy or milk protein ingestion in humans. The Journal of Nutrition, 133(5), 1308-1315.
- Brown, E. C., DiSilvestro, R. A., Babaknia, A., & Devor, S. T. (2004). Soy versus whey protein bars: effects on exercise training impact on lean body mass and antioxidant status. Nutrition Journal, 3(1), 22.
- FAO Report of a sub-committee of the 2011 FAO Consultation on “Protein Quality Evaluation in Human Nutrition”: the assessment of amino acid digestibility in foods for humans and including a collation of published ileal amino acid digestibility data for human foods. (2012). Rome, Italy.
- Fouillet, H., Juillet, B., Gaudichon, C., Mariotti, F., Tomé, D., & Bos, C. (2009). Absorption kinetics are a key factor regulating postprandial protein metabolism in response to qualitative and quantitative variations in protein intake. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 297(6), R1691-R1705.
- Fouillet, H., Mariotti, F., Gaudichon, C., Bos, C., & Tomé, D. (2002). Peripheral and splanchnic metabolism of dietary nitrogen are differently affected by the protein source in humans as assessed by compartmental modeling. The Journal of Nutrition, 132(1), 125-133.
- Hartman, J. W., Tang, J. E., Wilkinson, S. B., Tarnopolsky, M. A., Lawrence, R. L., Fullerton, A. V., & Phillips, S. M. (2007). Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. The American Journal of Clinical Nutrition, 86(2), 373-381.
- Joy, J. M., Lowery, R. P., Wilson, J. M., Purpura, M., De Souza, E. O., Wilson, S. M., … & Jäger, R. (2013). The effects of 8 weeks of whey or rice protein supplementation on body composition and exercise performance. Nutrition Journal, 12(1), 86.
- Koopman, R., Crombach, N., Gijsen, A. P., Walrand, S., Fauquant, J., Kies, A. K., … & van Loon, L. J. (2009). Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. The American Journal of Clinical Nutrition, 90(1), 106-115.
- Koopman, R., & van Loon, L. J. (2009). Aging, exercise, and muscle protein metabolism. Journal of Applied Physiology, 106(6), 2040-2048.
- Lenzi, J. L., Teixeira, E. L., de Jesus, G., Schoenfeld, B. J., & de Salles Painelli, V. (2019). Dietary strategies of modern bodybuilders during different phases of the competitive cycle. Journal of Strength and Conditioning Research.
- Leser, S. (2013). The 2013 FAO report on dietary protein quality evaluation in human nutrition: recommendations and implications. Nutrition Bulletin, 38(4), 421-428.
- Minevich, J., Olson, M. A., Mannion, J. P., Boublik, J. H., McPherson, J. O., Lowery, R. P., … & Purpura, M. (2015). Digestive enzymes reduce quality differences between plant and animal proteins: a double-blind crossover study. Journal of the International Society of Sports Nutrition, 12(1), P26.
- Paddon-Jones, D., Sheffield-Moore, M., Zhang, X. J., Volpi, E., Wolf, S. E., Aarsland, A., … & Wolfe, R. R. (2004). Amino acid ingestion improves muscle protein synthesis in the young and elderly. American Journal of Physiology-Endocrinology and Metabolism, 286(3), E321-E328.
- Paddon-Jones, D., Short, K. R., Campbell, W. W., Volpi, E., & Wolfe, R. R. (2008). Role of dietary protein in the sarcopenia of aging. The American Journal of Clinical Nutrition, 87(5), 1562S-1566S.
- Pennings, B., Boirie, Y., Senden, J. M., Gijsen, A. P., Kuipers, H., & van Loon, L. J. (2011). Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. The American Journal of Clinical Nutrition, 93(5), 997-1005.
- Phillips, S. M. (2012). Nutrient-rich meat proteins in offsetting age-related muscle loss. Meat Science, 92(3), 174-178.
- Phillips, S. M. (2011). The science of muscle hypertrophy: making dietary protein count. Proceedings of the Nutrition Society, 70(1), 100-103.
- Rogerson, D. (2017). Vegan diets: practical advice for athletes and exercisers. Journal of the International Society of Sports Nutrition, 14(1), 36.
- Soeters, P. B., & Deutz, N. (2001). The protein sparing function of the gut and the quality of food protein. Clinical Nutrition, 20(2), 97-99.
- Tang, J. E., Moore, D. R., Kujbida, G. W., Tarnopolsky, M. A., & Phillips, S. M. (2009). Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. Journal of Applied Physiology, 107(3), 987-992.
- Tipton, K. D., Ferrando, A. A., Phillips, S. M., Doyle Jr, D., & Wolfe, R. R. (1999). Postexercise net protein synthesis in human muscle from orally administered amino acids. American Journal of Physiology-Endocrinology And Metabolism, 276(4), E628-E634.
- Tipton, K. D., Gurkin, B. E., Matin, S., & Wolfe, R. R. (1999). Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. The Journal of Nutritional Biochemistry, 10(2), 89-95.
- Valente, L. M., Cabral, E. M., Sousa, V., Cunha, L. M., & Fernandes, J. M. (2016). Plant protein blends in diets for Senegalese sole affect skeletal muscle growth, flesh texture and the expression of related genes. Aquaculture, 453, 77-85.
- van Loon, L. J. (2012). Leucine as a pharmaconutrient in health and disease. Current Opinion in Clinical Nutrition & Metabolic Care, 15(1), 71-77.
- van Vliet, S., Burd, N. A., & van Loon, L. J. (2015). The skeletal muscle anabolic response to plant-versus animal-based protein consumption. The Journal of Nutrition, 145(9), 1981-1991.
- Venderley, A. M., & Campbell, W. W. (2006). Vegetarian diets. Sports Medicine, 36(4), 293-305.
- Volek, J. S., Volk, B. M., Gómez, A. L., Kunces, L. J., Kupchak, B. R., Freidenreich, D. J., … & Quann, E. E. (2013). Whey protein supplementation during resistance training augments lean body mass. Journal of the American College of Nutrition, 32(2), 122-135.
- Volpi, E., Kobayashi, H., Sheffield-Moore, M., Mittendorfer, B., & Wolfe, R. R. (2003). Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. The American Journal of Clinical Nutrition, 78(2), 250-258.
- Wilkinson, S. B., Tarnopolsky, M. A., MacDonald, M. J., MacDonald, J. R., Armstrong, D., & Phillips, S. M. (2007). Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. The American Journal of Clinical Nutrition, 85(4), 1031-1040.
- Wolfe, R. R. (2002). Regulation of muscle protein by amino acids. The Journal of Nutrition, 132(10), 3219S-3224S.
- Yang, Y., Churchward-Venne, T. A., Burd, N. A., Breen, L., Tarnopolsky, M. A., & Phillips, S. M. (2012). Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutrition & Metabolism, 9(1), 57.
From being a mediocre athlete, to professional powerlifter and strength coach, and now to researcher and writer, Charlie combines education and experience in the effort to help Bridge the Gap Between Science and Application. Charlie performs double duty by being the Content Manager for The Muscle PhD as well as the Director of Human Performance at the Applied Science and Performance Institute in Tampa, FL. To appease the nerds, Charlie is a PhD candidate in Human Performance with a master’s degree in Kinesiology and a bachelor’s degree in Exercise Science. For more alphabet soup, Charlie is also a Certified Strength and Conditioning Specialist (CSCS), an ACSM-certified Exercise Physiologist (ACSM-EP), and a USA Weightlifting-certified performance coach (USAW).




