Introduction
The calves are probably the trickiest muscle group for most bodybuilders to develop. This has spurned many a social media trend, such as #teamnocalves, etc. We even hear many people throwing in the towel on calf training, claiming that they simply don’t have the genetics for big calves. While genetics can play a massive role in muscle development, there’s still some secrets to calf training that are worth discussing more in detail.
Unfortunately for many lifters, there’s very little scientific data on calf training. Mostly because nobody cares to perform and/or fund research on calf muscle hypertrophy. Therefore, uncovering more information about potential calf training clues involves diving into rehab journals, analyzing walking gaits, and even information about rats and cats since we know relatively little about the human calf muscles.
Even with that being said, we’ve compiled enough findings here to kickstart one’s calf training journey. So, without further ado, let’s discuss the ins-and-outs of calf training.
Anatomy of the Calves
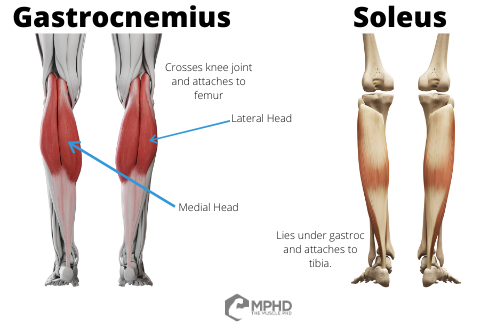 When discussing how to best train a muscle group, where’s the first place you should look for clues? Anatomy. Anatomy can tell us many things about how to train a muscle – how large the muscle is, where its origin and insertion are, what joints it crosses, and even the pennation angle of its muscle fibers. With that being said, let’s go over some basic anatomy of the calves.
When discussing how to best train a muscle group, where’s the first place you should look for clues? Anatomy. Anatomy can tell us many things about how to train a muscle – how large the muscle is, where its origin and insertion are, what joints it crosses, and even the pennation angle of its muscle fibers. With that being said, let’s go over some basic anatomy of the calves.
The calf muscles are collectively known as the triceps surae, since there’s 3 unique muscle heads in this group. The two main muscles involved are the soleus, which lies under the other calf muscle, the gastrocnemius. The gastrocnemius can be split into both the lateral and medial gastroc which lends us the 3 head designation (28). The medial and lateral gastroc are the main muscles you see when you hit a calf pose (assuming you don’t have bird legs) – the soleus lies deep and doesn’t contribute much to the detail of the calves besides adding thickness to your lower leg.
Now, let’s get to some more important anatomy components. First off, the big one – the soleus is a single joint muscle in that it originates on the back of the tibia and inserts into the Achilles tendon. The Achilles tendon then crosses the heel to perform actions at the ankle joint – we’ll cover that more in the next section. However, the gastroc is a little different. The gastroc originates on the femur and inserts into the Achilles tendon (28). Therefore, the gastroc crosses both the knee and ankle joints. This can have implications for training that we’ll get to in the next section.
Actions of the Calves
We know that both calf muscles act on the ankle joint – they primarily perform plantar flexion which occurs when you point your toes. The calves can also be involved in other ankle joint actions, such as eversion and inversion (28), but I doubt many people are performing those exercises in an effort to stimulate calf growth. Lastly, the calves will undergo a large stretch during ankle dorsiflexion, which occurs when you bring your toes to your knees. This can also have implications for calf training that we’ll get into in another section.
The calves are also active during things like walking, balancing, and basically anything else that involves standing. However, the calves are extremely efficient during walking (20) and are unlikely to get much of a training stimulus out of walking. In addition, the calves are actually decently active during things like squats (14,26,32,35) and deadlifts (3,13) and have even been shown to grow slightly from a flywheel device squat program (30).
So, from this section, we can see that calf raises are probably the best way to target the calves since calf raises are pretty much the only exercise that isolates ankle plantar flexion. Since that’s not news to anyone, let’s dig a bit deeper.
Muscle Size and Training Considerations
The next stop we take when trying to figure out how to train a muscle is the general size of the muscle. This is often reflected in findings concerning either muscle cross-sectional area or muscle volume as simply looking at a muscle doesn’t tell the whole story. This is important, since many would incorrectly assume muscle size based off of the eye test – we see this reflected in the fact that the triceps are technically a larger muscle than the lats (22) since the lats are, realistically, a pretty thin muscle. Pretty sure no one would have guessed that by simply glancing at someone.
 So why is muscle size important? Muscle size typically tells us how easily we can activate a muscle. Muscle activation is important because it can tell us roughly half of the story as to whether or not a muscle will grow from a given exercise. A muscle certainly needs to be active to grow, but it’s also going to need to be mechanically challenged, which we will get to in another section. Regardless, we know that, generally, the larger a muscle is, the harder it’s going to be to maximally activate. This is reflected in the findings that the quads are one of the hardest muscle groups to activate (2) whereas the biceps are probably the easiest major muscle group to activate (11).
So why is muscle size important? Muscle size typically tells us how easily we can activate a muscle. Muscle activation is important because it can tell us roughly half of the story as to whether or not a muscle will grow from a given exercise. A muscle certainly needs to be active to grow, but it’s also going to need to be mechanically challenged, which we will get to in another section. Regardless, we know that, generally, the larger a muscle is, the harder it’s going to be to maximally activate. This is reflected in the findings that the quads are one of the hardest muscle groups to activate (2) whereas the biceps are probably the easiest major muscle group to activate (11).
Why is activation important? Besides being a harbinger for potential growth, activation can also tell us how much muscle damage we might expect from training. This is also dependent on mechanical factors, but again, we have research showing that larger muscle groups typically recover from damaging exercise more quickly (6) because they don’t activate as well and don’t get as damaged/sore.
So how do the calves stack up? The gastroc muscle is actually pretty large relative to other muscles in the body; it’s obviously not as large as the quads, but it is definitely bigger than the biceps – in fact, just the medial head of the gastroc alone is probably larger than the biceps (8). The lateral head of the gastroc and the entire soleus muscle are a little smaller than the medial gastroc (8) and, therefore, are probably easier to activate. Frankly, this sucks, because the medial gastroc makes up the bulk of our visible calves, but it’s probably going to be the hardest muscle in the calves to activate.
With all of this in mind, the calves aren’t the most difficult muscle to activate, but they certainly don’t activate as well as smaller muscles (15). Therefore, the calves probably won’t get as sore as many upper body muscles, but they’ll also likely recover quicker which can offer more clues for training.
Muscle Fiber Type and Training Considerations
Following an assessment of muscle size, we then need to uncover the fiber type composition of a muscle to determine more training factors. Fiber type composition can tell us three things about a muscle – how susceptible it is to getting damaged/sore, how much growth we can expect, and what repetition ranges might be best for training it. So, how do the calves stack up?
As far as the gastroc goes, science has uncovered a wide range of possible fiber splits, all of the way from 44% slow twitch to 76% slow twitch (9,10,12,24). The soleus, on the other hand, has been consistently shown to be very slow twitch, with a range between 70% and 96% slow twitch fibers being present (9,10,12,24). With that being said, the gastroc is probably going to be a little on the slower side for most people unless you’re a pretty good sprinter. However, the soleus is definitely going to be on the slower side, no matter your athletic abilities. Let’s discuss what this means for training.
First off, fast twitch muscle fibers are more likely to experience muscle damage than slow twitch muscle fibers (17) and this is likely going to be due to fast twitch fibers developing more force as well as being less oxidative than slow twitch fibers. With this in mind, the calves probably won’t get very damaged or sore from training, and we see this reflected in the fact that the calves are one of the fastest muscle groups to recover following damaging training (6). While not being sore often feels like you didn’t do enough, this does offer a clue on training frequency for the calves. Since the calves aren’t damaged much from training and recover pretty quickly, you can probably train the calves relatively often. There’s hint #1.
Secondly, knowing fiber type composition of a muscle can somewhat help us predict how well it will grow from training. Fast twitch muscle fibers will grow the most from normal training (31) since these fibers produce the most force and experience the most fatigue and tension during conventional training. Since the calves have a large slow twitch percentage, this means that sticking to the classic 8-12 rep range might not always be the best method for the calves. There’s hint #2.
Let’s go ahead and cover hint #2 more in-depth. Fiber type composition can help us decide how much time to spend using certain rep ranges. Since the calves are mostly slow twitch, how does that information guide this decision? We know that slow twitch muscle fibers are very resistant to fatigue. Therefore, these muscle fibers likely require a lot more time under tension in order to grow (16). How do you increase time under tension? Do more reps. Going slow on purpose will simply reduce muscle activation and won’t cause much growth – check our Time Under Tension article (here) for more info on that.
So, the muscle fiber type composition of the calves tells us that we can train the calves pretty often and should also probably use a good amount of volume to maximize growth. We’ve got one last component to uncover.
Muscle and Joint ROM and Training Considerations
 Joint range of motion is the last component to consider when attempting to develop a training plan for a muscle group. This range of motion can impact how much stretch a muscle experiences during normal training which can then mediate muscle damage, soreness, and overall recovery needs. Since the calves primarily act on the ankle joint, what information can we gather?
Joint range of motion is the last component to consider when attempting to develop a training plan for a muscle group. This range of motion can impact how much stretch a muscle experiences during normal training which can then mediate muscle damage, soreness, and overall recovery needs. Since the calves primarily act on the ankle joint, what information can we gather?
First off, the ankle joint doesn’t go through a large range of motion; it’s a decently restricted joint that simply doesn’t need to perform a wide range of motion. With this in mind, it’s highly unlikely that normal ankle range of motion can induce much of a stretch on the calf muscles, let alone enough stretch to cause much damage or soreness.
This is unfortunate, because the individual contractile units within the soleus can actually stretch to large lengths (5) which means that the soleus might get a better training stimulus from larger range of motion movements. The contractile units in the gastroc don’t stretch quite as far (19,21,27), so if you have any soreness from training, it’s probably in your gastroc since the stretching of these units can cause damage and soreness (4). This is useful information because we know that loaded stretching can induce growth (25), so focusing on the stretch as much as possible during calf exercises might be one small key to calf gains.
However, ankle range of motion can still play a role in this scenario. Unfortunately, both tight calf muscles and even a tight sciatic nerve can contribute to reducing ankle range of motion (1). With this in mind, it’s important to perform a full warm-up to ensure that your ankles are ready to undergo loaded stretching. Even if it’s only a few additional degrees of ROM, that small extra range can help growth in the long term.
Another major component of joint ROM to cover is the difference between seated and standing calf raises. These will result in similar actions at the ankle joint, but flexing the knee in seated calf raises has some interesting effects on calf muscle activation. This is pretty well-known by now, but flexing the knee reduces gastroc activation (7,18,23) and increases soleus activation (29,33,36). This is mostly due to a phenomenon known as “active insufficiency.” Since the gastroc crosses both the knee and the ankle joint, flexing the knee shortens the gastroc to a large extent which leaves it mostly incapable of plantar flexing the ankle. Since the gastroc starts to shut down with the knees flexed, the soleus has to take the brunt of the force.
Lastly, can pointing your toes in different directions (and thus altering ankle angle) change calf activation? We really don’t have much clear data on this, we mostly have to rely on the mechanical advantage of the calf muscles in each position of toe angle. With this in mind, pointing your toes out will probably emphasize the medial gastroc whereas pointing the toes in should emphasize the lateral gastroc. It’s important to note that the ankle should be able to undergo its greatest dorsiflexion ROM in a pretty neutral position (37), so you probably won’t get as much of a stretch in the calves when pointing the toes in one direction or the other.
Update on the previous paragraph! A recent study investigated the toe angle question and we have an entire Quick Tip dedicated to that study here. In short, calf raises with the toes pointed out do develop the inner calf more whereas pointing the toes out develops the outer calf more.
Takeaways
So, how can we put all of this knowledge together? Let’s sum it up in a list:
- The calf muscles aren’t activated as easily as many upper body muscles. This means that they’re less likely to be damaged and/or sore from exercise.
- The calf muscles are mostly slow twitch muscle fibers, meaning that (again), they’re less likely to be damaged and/or sore than other muscle groups.
- The ankle joint doesn’t go through a large range of motion, so it can be difficult to get a big stretch in the calves. Holding the stretched position in calf raises might be a way to overcome this issue.
With these three main findings in mind, we can infer these key takeaways:
- Since the calves are not readily damaged from training, you can probably train them quite often. We’d recommend 4-5 days a week – this can be done by simply adding in a calf raise variation at the end of almost every workout you perform throughout the week.
- Make sure you’re hitting a full range of motion during every calf raise exercise – especially emphasizing the stretched position. The ankle is incredibly mechanically efficient and the Achilles tendon can store a large amount of elastic energy. With these issues in mind, pausing in the stretched position of calf raises is likely necessary to maximize calf growth.
- Include calf raise variations that involve a straight leg to target the gastroc and a bent leg to target the soleus. Since the gastroc makes up the majority of your calves, spend at least 60% of your total calf training volume on the gastroc.
- Lastly, due to the slow twitch nature of the calves, performing higher reps is likely necessary to force growth in these muscles. The slow twitch fibers are going to be resistant to fatigue and likely require a large time under tension in order to grow. Therefore, the majority of your calf training should fall into the 15-30 reps per set range, while a smaller amount (maybe 1-2 workouts per week) can sit in the 6-12 rep range.
Calf training can be incredibly frustrating, especially to those whom are not genetically endowed with watermelons in their lower legs. We’ve seen some recommend simply adding 100 reps of calf raises at the end of every workout to stimulate new calf growth. While that’s certainly one strategy that aligns with our recommendations, it’s much more worthwhile to devote a 2500+ word article covering why that might be effective. All-in-all, we hope that this piece can serve as a guide for leading your calves out to pasture to maximize growth.
Man, we almost made it through the entire article without a cow pun. We tried.
References
- Andrade, R. J., Freitas, S. R., Hug, F., Le Sant, G., Lacourpaille, L., Gross, R., … & Nordez, A. (2018). The potential role of sciatic nerve stiffness in the limitation of maximal ankle range of motion. Scientific Reports, 8(1), 1-10.
- Beltman, J. G. M., Sargeant, A. J., Van Mechelen, W., & De Haan, A. (2004). Voluntary activation level and muscle fiber recruitment of human quadriceps during lengthening contractions. Journal of Applied Physiology, 97(2), 619-626.
- Bezerra, E. S., Simão, R., Fleck, S. J., Paz, G., Maia, M., Costa, P. B., & Serrão, J. C. (2013). Electromyographic Activity of Lower Body Muscles during the Deadlift and Still-Legged Deadlift. Journal of Exercise Physiology Online, 16(3).
- Brockett, C. L., Morgan, D. L., Gregory, J. E., & Proske, U. (2002). Damage to different motor units from active lengthening of the medial gastrocnemius muscle of the cat. Journal of Applied Physiology, 92(3), 1104-1110.
- Chen, X., & Delp, S. L. (2016). Human soleus sarcomere lengths measured using in vivo microendoscopy at two ankle flexion angles. Journal of Biomechanics, 49(16), 4164-4167.
- Chen, T. C., Yang, T. J., Huang, M. J., Wang, H. S., Tseng, K. W., Chen, H. L., & Nosaka, K. (2019). Damage and the repeated bout effect of arm, leg, and trunk muscles induced by eccentric resistance exercises. Scandinavian Journal of Medicine & Science in Sports, 29(5), 725-735.
- Cresswell, A. G., Löscher, W. N., & Thorstensson, A. (1995). Influence of gastrocnemius muscle length on triceps surae torque development and electromyographic activity in man. Experimental Brain Research, 105(2), 283-290.
- Crouzier, M., Lacourpaille, L., Nordez, A., Tucker, K., & Hug, F. (2018). Neuromechanical coupling within the human triceps surae and its consequence on individual force-sharing strategies. Journal of Experimental Biology, 221(21).
- Dahmane, R., Djordjevic, S., & Smerdu, V. (2007). Adaptive potential of human biceps femoris muscle demonstrated by histochemical, immunohistochemical and mechanomygraphical methods. Medical and Biological Engineering and Computing, 45(3), 323-324.
- Dahmane, R., Djordjevič, S., Šimunič, B., & Valenčič, V. (2005). Spatial fiber type distribution in normal human muscle: histochemical and tensiomyographical evaluation. Journal of Biomechanics, 38(12), 2451-2459.
- De Serres, S. J., & Enoka, R. M. (1998). Older adults can maximally activate the biceps brachii muscle by voluntary command. Journal of Applied Physiology, 84(1), 284-291.
- Edgerton, V. R., Smith, J. L., & Simpson, D. R. (1975). Muscle fibre type populations of human leg muscles. The Histochemical Journal, 7(3), 259-266.
- Escamilla, R. F., Francisco, A. C., Kayes, A. V., Speer, K. P., & Moorman 3rd, C. T. (2002). An electromyographic analysis of sumo and conventional style deadlifts. Medicine & Science in Sports & Exercise, 34(4), 682-688.
- Gorsuch, J., Long, J., Miller, K., Primeau, K., Rutledge, S., Sossong, A., & Durocher, J. J. (2013). The effect of squat depth on multiarticular muscle activation in collegiate cross-country runners. The Journal of Strength & Conditioning Research, 27(9), 2619.
- Gondin, J., Guette, M., Maffiuletti, N. A., & Martin, A. (2004). Neural activation of the triceps surae is impaired following 2 weeks of immobilization. European Journal of Applied Physiology, 93(3), 359-365.
- Grgic, J., Homolak, J., Mikulic, P., Botella, J., & Schoenfeld, B. J. (2018). Inducing hypertrophic effects of type I skeletal muscle fibers: A hypothetical role of time under load in resistance training aimed at muscular hypertrophy. Medical Hypotheses, 112, 40-42.
- Friden, J. (1992). Structual and mechanical basis of exercise-induced muscle injury. Medicine and Science in Sports and Exercise, 16, 456-459.
- Hébert-Losier, K., Schneiders, A. G., García, J. A., Sullivan, S. J., & Simoneau, G. G. (2012). Influence of knee flexion angle and age on triceps surae muscle activity during heel raises. The Journal of Strength & Conditioning Research, 26(11), 3124-3133.
- Herzog, W., Read, L. J., & Ter Keurs, H. E. D. J. (1991). Experimental determination of force—length relations of intact human gastrocnemius muscles. Clinical Biomechanics, 6(4), 230-238.
- Hof, A. L., Geelen, B. A., & Van den Berg, J. W. (1983). Calf muscle moment, work and efficiency in level walking; role of series elasticity. Journal of Biomechanics, 16(7), 523-537.
- Hoffman, B. W., Cresswell, A. G., Carroll, T. J., & Lichtwark, G. A. (2013). Muscle fascicle strains in human gastrocnemius during backward downhill walking. American Journal of Physiology-Heart and Circulatory Physiology.
- Holzbaur, K. R., Murray, W. M., Gold, G. E., & Delp, S. L. (2007). Upper limb muscle volumes in adult subjects. Journal of Biomechanics, 40(4), 742-749.
- Jennekens, F. G., Tomlinson, B. E., & Walton, J. N. (1971). Data on the distribution of fibre types in five human limb muscles. An autopsy study. Journal of the Neurological Sciences, 14(3), 245.
- Johnson, M., Polgar, J., Weightman, D., & Appleton, D. (1973). Data on the distribution of fibre types in thirty-six human muscles: an autopsy study. Journal of the Neurological Sciences, 18(1), 111-129.
- Krüger, M., & Kötter, S. (2016). Titin, a central mediator for hypertrophic signaling, exercise-induced mechanosignaling and skeletal muscle remodeling. Frontiers in Physiology, 7, 76.
- Maddigan, M. E., Button, D. C., & Behm, D. G. (2014). Lower-Limb and Trunk Muscle Activation With Back Squats and Weighted Sled Apparatus. The Journal of Strength & Conditioning Research, 28(12), 3346-3353.
- Maganaris, C. N. (2003). Force‐length characteristics of the in vivo human gastrocnemius muscle. Clinical Anatomy: The Official Journal of the American Association of Clinical Anatomists and the British Association of Clinical Anatomists, 16(3), 215-223.
- Marieb, E. N., Wilhelm, P. B., & Mallatt, J. (2014). Human Anatomy (Vol. 784). Pearson.
- Price, T. B., Kamen, G., Damon, B. M., Knight, C. A., Applegate, B., Gore, J. C., & Signorile, J. F. (2003). Comparison of MRI with EMG to study muscle activity associated with dynamic plantar flexion. Magnetic Resonance Imaging, 21(8), 853-861.
- Sanz-López, F., Berzosa Sánchez, C., Hita-Contreras, F., Cruz-Diaz, D., & Martínez-Amat, A. (2016). Ultrasound changes in achilles tendon and gastrocnemius medialis muscle on squat eccentric overload and running performance. Journal of Strength and Conditioning Research, 30(7), 2010-2018.
- Sale, D. G. (1987). Influence of exercise and training on motor unit activation. Exercise and Sport Sciences Reviews, 15, 95-151.
- Schwanbeck, S., Chilibeck, P. D., & Binsted, G. (2009). A comparison of free weight squat to Smith machine squat using electromyography. The Journal of Strength & Conditioning Research, 23(9), 2588-2591.
- Signorile, J. E., Applegate, B., Ducque, M., Cole, N., & Zink, A. (2002). Selective recruitment of the triceps surae muscles with changes in knee angle. The Journal of Strength & Conditioning Research, 16(3), 433-439.
- Sinclair, J., McCarthy, D., Bentley, I., Hurst, H. T., & Atkins, S. (2014). The influence of different footwear on 3-D kinematics and muscle activation during the barbell back squat in males. European Journal of Sport Science, 1.
- Slater, L. V., & Hart, J. M. (2017). Muscle activation patterns during different squat techniques. Journal of Strength and Conditioning Research, 31(3), 667-676.
- Tamaki, H., Kitada, K., Akamine, T., Sakou, T., & Kurata, H. (1996). Electromyogram patterns during plantarflexions at various angular velocities and knee angles in human triceps surae muscles. European Journal of Applied Physiology and Occupational Physiology, 75(1), 1-6.
- Tiberio, D. (1987). Evaluation of functional ankle dorsiflexion using subtalar neutral position: a clinical report. Physical Therapy, 67(6), 955-957.
From being a mediocre athlete, to professional powerlifter and strength coach, and now to researcher and writer, Charlie combines education and experience in the effort to help Bridge the Gap Between Science and Application. Charlie performs double duty by being the Content Manager for The Muscle PhD as well as the Director of Human Performance at the Applied Science and Performance Institute in Tampa, FL. To appease the nerds, Charlie is a PhD candidate in Human Performance with a master’s degree in Kinesiology and a bachelor’s degree in Exercise Science. For more alphabet soup, Charlie is also a Certified Strength and Conditioning Specialist (CSCS), an ACSM-certified Exercise Physiologist (ACSM-EP), and a USA Weightlifting-certified performance coach (USAW).




