Introduction
Throughout several articles on this site we discuss the importance of an optimal training program – essentially a solid training plan with both diet and proper rest accounted for. However, we haven’t really discussed the biological basis for why there is an optimum amount of all of these things, so let’s dig a little deeper into that discussion this time.
What is Hormesis?
16th century Swiss physician and alchemist, Paracelcus, is noted for saying, “Solely the dose determines that a thing is not a poison” (4). Essentially, our ancient friend is referring to the idea that any particular substance is not harmful up to a certain dose, so even cyanide, for instance, could be theoretically safe up to a point. Later scientists involved in toxicology termed this idea, “hormesis,” and stated that an organism will respond to a particular substance or stressor in a bell curve fashion (26). So, the idea of Paracelcus is not far off – a too small dose of cyanide may not cause a severe biological response, but a dose that is just right, well, does its thing. RIP tons of political figures throughout history.
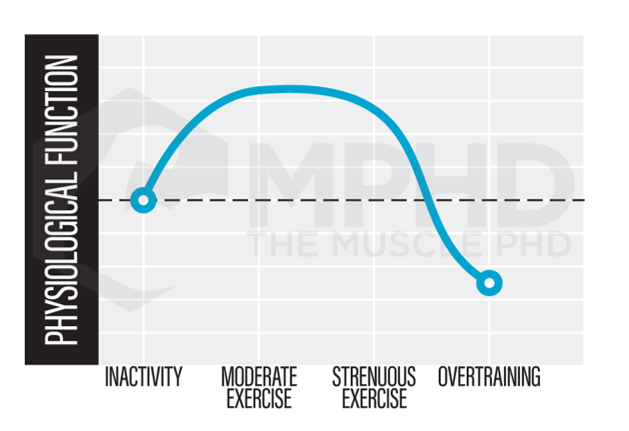 Hormesis, then, can directly relate to exercise as shown in the bell curve to the right. Too little exercise does not offer a significant enough stress for adaptation and if that idea gets taken too far, a completely sedentary lifestyle can increase incidence of various diseases and lead to early death (3). On the other far end of the spectrum, we have exercise that is too intense and accompanied by improper nutrition and poor rest. This end of the spectrum is also associated with increased incidence in illness and decreases in physical performance (21). Check out our Training and Illness article (here) for more info.
Hormesis, then, can directly relate to exercise as shown in the bell curve to the right. Too little exercise does not offer a significant enough stress for adaptation and if that idea gets taken too far, a completely sedentary lifestyle can increase incidence of various diseases and lead to early death (3). On the other far end of the spectrum, we have exercise that is too intense and accompanied by improper nutrition and poor rest. This end of the spectrum is also associated with increased incidence in illness and decreases in physical performance (21). Check out our Training and Illness article (here) for more info.
Hormesis and Bodybuilding
By examining the hormesis curve, it’s rather obvious that maintaining a training stress within the optimal region of the bell curve will lead to greater gains from exercise. We see a primary example of this in a recent meta-analysis on the dose-response relationship between training volume and gains. It was found that increasing training volume to at least 10-sets per body part per week was more effective for increasing muscle size and strength than protocols with fewer than 10-sets per body part per week (31). On the other hand, other studies show that extremely intense exercise can actually lead to cell degradation and even cell death (1). These findings clearly imply an optimal middle ground for training gains.
Researchers have noted that there certainly is an optimal range for a training stimulus (5,23), especially when considering the hormesis curve, but it’s tough to identify what exactly that range is – especially when we consider the individuality of training gains. We already discussed the idea of training volume, but it’s important to understand that overall gains in muscle size are predicated on the balance between protein synthesis and protein breakdown (30). Intense training can increase levels of both protein synthesis and protein breakdown (7), so it’s also necessary to maximize nutrition strategies to stimulate greater protein synthesis and achieve an overall positive protein balance – which is necessary for muscle growth (25). Employing proper nutrition and rest protocols can assist in maintaining an optimal position on the hormesis curve when undergoing intense training. Without optimizing both nutrition and rest, you’re much more likely to push towards the far end of the curve and may even end up overtraining.
Now, on to the more fun and slightly more theoretical component of hormesis and bodybuilding. Some scientists theorize that certain recovery methods may actually bring about an additional stimulus that can help maintain an optimal position on the hormesis curve (23). I highly recommend digging through the comprehensive review by Peake et al. (2015) that is often cited throughout this piece if you’re interested in more information. Essentially, the idea behind some of these interventions is to avoid the back-and-forth on the bell curve – spending time on one extreme end during intense training and then reverting to the opposite side when resting and doing nothing. Altering carbohydrate intake, performing blood flow restriction, and hot and cold therapies have all been proposed as potential methods to offer further stimulus besides training which may help maintain the sweet spot on the hormesis curve (23).
Carbohydrate Restriction
One very interesting nutrition-based theory is that carbohydrate-restricted diets may actually induce further stimulus on the muscle to promote exercise adaptations. Lower muscle glycogen levels from carbohydrate-restricted diets may impose an additional small stressor to the muscle, one that might just be enough to optimize adaptation when concerning the hormesis curve. We see potential evidence of a benefit from this method in studies that find improved aerobic enzyme (11,19,45), AMPK activity (41,44), and fat metabolism (14) in subjects undergoing exercise with low muscle glycogen stores. AMPK upregulation may impact several signaling pathways that include glucose transport, fatty acid uptake, and mitochondrial biogenesis (12). All of these alterations could lead to increased fat burning following exercise, however, AMPK activation will also likely impair strength and size gains, so this strategy would be 100% dependent on your goals.
 In addition, training at low muscle glycogen levels may also increase protein breakdown (2,13) so this whole idea represents an interesting scenario in science. On one hand, we have potential benefits, but on the other hand, we see either no effect or even negative effects. This is one of those situations where human variation and individual goals can play a massive role. If you simply want to build as much muscle as possible, extreme carbohydrate restriction (without going full keto) may not be your best strategy.
In addition, training at low muscle glycogen levels may also increase protein breakdown (2,13) so this whole idea represents an interesting scenario in science. On one hand, we have potential benefits, but on the other hand, we see either no effect or even negative effects. This is one of those situations where human variation and individual goals can play a massive role. If you simply want to build as much muscle as possible, extreme carbohydrate restriction (without going full keto) may not be your best strategy.
More research certainly needs to be done in this arena as we don’t have enough answers on how severe carbohydrate restriction might affect long term strength and size gains (in non-keto folks, at least). Therefore, if you choose to use this strategy for achieving optimal hormesis, don’t be afraid to play around with carb intake and timing until you find what works best for you.
If you’re interested in more info on exercise adaptations to carbohydrate restriction, check out our Carb Cycling article (here). And, if you’re interested in more info about carbs and bodybuilding in general, check out our Nutrient Timing article here.
Blood Flow Restriction
Blood flow restriction training is a great way to add variation into your training and may actually represent an interesting scenario with hormesis in mind. Blood flow restriction training offers similar gains to traditional training with heavier loads but without the large amounts of muscle damage that can impair recovery (18). This places BFR training in the sweet spot of the hormesis curve due to the fact that it offers a great stimulus without inducing a ton of damage or requiring a ton of intensity.
BFR may be able to match up to traditional heavy training due to five main findings:
1) BFR can increase motor unit recruitment due to an increased rate of fatigue during a set (39). More motor unit recruitment means that more fibers are experiencing mechanical tension which is a huge determinant of growth (38).
2) BFR may increase the growth hormone response to exercise (36). While this response may not have significant impacts on muscle growth, growth hormone can significantly increase matrix collagen synthesis (6) which can improve connective tissue strength and health. It’s tough to say whether or not natural increases in hormone production will significantly influence long term gains (more info here), but it’s certainly an interesting difference between BFR and traditional training.
3) BFR can increase the oxidative stress incurred during exercise (39). Oxidative stress may act as a growth signal following exercise (23), so increasing this stress may be one route through which light training with BFR can match up to traditional heavy training.
4) BFR may increase cell swelling due to blood pooling and metabolite accumulation (17). Cell swelling may exert mechanical tension on the cell in a unique manner (38) which might be why we see similar gains from BFR and traditional training.
5) BFR may increase satellite cell activation following exercise (40). Increased satellite cell activation is correlated with greater muscle growth in a training protocol (24).
Therefore, adding BFR training to your program can be a great way to add variation without pushing the overall training stimulus too far towards the overtraining end of the hormesis curve. Using BFR methods without training may also induce some of the above benefits, so intermittently wrapping limbs during rest days or periods of injury could have some benefits as well.
Heat Application
 An increase in muscle tissue temperature is one of the main side effects of exercise, so it’s been theorized that passively heating muscles may impart a small stimulus that could assist in maintaining optimal hormesis. One of the main ways that heat application may help promote gains is through simply increasing blood flow to the muscle (33,42). An improvement in blood flow can increase the amount of oxygen and nutrients that are delivered to the muscle (9), both of which can greatly impact recovery.
An increase in muscle tissue temperature is one of the main side effects of exercise, so it’s been theorized that passively heating muscles may impart a small stimulus that could assist in maintaining optimal hormesis. One of the main ways that heat application may help promote gains is through simply increasing blood flow to the muscle (33,42). An improvement in blood flow can increase the amount of oxygen and nutrients that are delivered to the muscle (9), both of which can greatly impact recovery.
Several rodent studies have found positive effects from using heat therapies before exercise. Applying heat before exercise may increase protein synthesis (10), reduce muscle fiber damage (35), and reduce mitochondrial damage (8).
Similar benefits have been shown in human studies, including increased satellite cell recruitment (16), reduced muscle fatigue (15), faster recovery of strength and range of motion (20), and alleviated muscle soreness (29).
How does one “pre-heat” before exercise? Most of the rodent studies used warm baths for up to 24-hours before exercise. As great as that sounds, it’s not realistic for most people so you’re probably better off focusing on a proper warm-up that increases tissue temperature before exercise. This includes things like plyometrics, biking, sprints, and even passive methods, like using your car’s seat warmers on the way to the gym (my personal favorite). The more realistic option is to try out heat therapies after exercise as they appear to offer the same benefits as pre-heating interventions. This includes warm baths, heat packs, and even saunas. Again, the heat may represent a low-grade stimulus that helps you maintain an optimal position on the hormesis bell curve.
Cold Therapies
The last potential mechanism that may offer some sort of stimulus is cryotherapy. Cryotherapy is anything that involves colder than physiological temperatures – the most common form being an ice bath. Multiple studies have shown that cold water immersion more than likely impairs adaptations to resistance training (22,27,43). This is probably due to the fact the colder temperatures will decrease blood flow to the muscle and can also reduce inflammation which acts as a growth signal following training. Cryotherapies may also reduce satellite cell recruitment (23) so it appears that these methods may not be the best hormesis-related intervention for overall gains.
One key takeaway from cryotherapy methods is that they may be useful if you’re performing more than one training session in a day. Taking an ice bath after your first session may improve performance in your second session (27). In this instance, you could take an ice bath after your first workout then use heat therapies after the second to kick start the next recovery process. That’s an interesting scenario that definitely requires further investigation. However, these findings have been isolated to endurance training, so bodybuilders or strength athletes might be better off avoiding cryotherapy all together.
Conclusion
The concept of hormesis is incredibly interesting and represents the necessity for a balanced training plan. Training that is too intense without proper diet or rest pushes one to the far end of the hormesis curve – the area associated with reduced training adaptations and increased incidence of illness. The other side of the curve is simply inactivity. If you don’t do anything, of course you’re not going to make any gains and you’re probably going to be generally unhealthy. Therefore, it’s imperative to find a way to keep yourself within the optimum adaptation range shown on the hormesis curve. Optimizing your training, nutrition, and rest day protocols are great ways to do this, while utilizing some of the recovery methods mentioned here may improve growth beyond just diet and rest alone.
Exercise and hormesis is still a relatively new topic, so keep your eyes out for new information on the subject. Hopefully this piece gives some good background information to many of the recommendations we make over and over again.
References
- Armand, A. S., Launay, T., Gaspera, B. D., Charbonnier, F., Gallien, C. L., & Chanoine, C. (2003). Effects of eccentric treadmill running on mouse soleus: degeneration/regeneration studied with Myf‐5 and MyoD probes. Acta Physiologica Scandinavica, 179(1), 75-84.
- Blomstrand, E., & Saltin, B. (1999). Effect of muscle glycogen on glucose, lactate and amino acid metabolism during exercise and recovery in human subjects. The Journal of Physiology, 514(1), 293-302.
- Booth, F. W., & Lees, S. J. (2007). Fundamental questions about genes, inactivity, and chronic diseases. Physiological Genomics, 28(2), 146-157.
- Borzelleca, J. F. (2000). Paracelsus: herald of modern toxicology. Toxicological Sciences, 53(1), 2-4.
- Calabrese, E. J., Bachmann, K. A., Bailer, A. J., Bolger, P. M., Borak, J., Cai, L., … & Cook, R. R. (2007). Biological stress response terminology: integrating the concepts of adaptive response and preconditioning stress within a hormetic dose–response framework. Toxicology and Applied Pharmacology, 222(1), 122-128.
- Doessing, S., Heinemeier, K. M., Holm, L., Mackey, A. L., Schjerling, P., Rennie, M., … & Flyvbjerg, A. (2010). Growth hormone stimulates the collagen synthesis in human tendon and skeletal muscle without affecting myofibrillar protein synthesis. The Journal of Physiology, 588(2), 341-351.5
- Fielding, R. A., Meredith, C. N., O’Reilly, K. P., Frontera, W. R., Cannon, J. G., & Evans, W. J. (1991). Enhanced protein breakdown after eccentric exercise in young and older men. Journal of Applied Physiology, 71(2), 674-679.
- Garramone, J. R., Winters, R. M., Das, D. K., & Deckers, P. J. (1994). Reduction of skeletal muscle injury through stress conditioning using the heat-shock response. Plastic and Reconstructive Surgery, 93(6), 1242-1247.
- Giombini, A., Giovannini, V., Cesare, A. D., Pacetti, P., Ichinoseki-Sekine, N., Shiraishi, M., … & Maffulli, N. (2007). Hyperthermia induced by microwave diathermy in the management of muscle and tendon injuries. British Medical Bulletin, 83(1), 379-396.
- Goto, K., Okuyama, R., Sugiyama, H., Honda, M., Kobayashi, T., Uehara, K., … & Yoshioka, T. (2003). Effects of heat stress and mechanical stretch on protein expression in cultured skeletal muscle cells. Pflügers Archiv, 447(2), 247-253.
- Hansen, A. K., Fischer, C. P., Plomgaard, P., Andersen, J. L., Saltin, B., & Pedersen, B. K. (2005). Skeletal muscle adaptation: training twice every second day vs. training once daily. Journal of Applied Physiology, 98(1), 93-99.
- Hardie, D. G. (2014). AMP-activated protein kinase: maintaining energy homeostasis at the cellular and whole-body levels. Annual Review of Nutrition, 34, 31-55.
- Howarth, K. R., Phillips, S. M., MacDonald, M. J., Richards, D., Moreau, N. A., & Gibala, M. J. (2010). Effect of glycogen availability on human skeletal muscle protein turnover during exercise and recovery. Journal of Applied Physiology, 109(2), 431-438.
- Hulston, C. J., Venables, M. C., Mann, C. H., Martin, C., Philp, A., Baar, K., & Jeukendrup, A. E. (2010). Training with low muscle glycogen enhances fat metabolism in well-trained cyclists. Medicine and Science in Sports and Exercise, 42(11), 2046-2055.
- Iguchi, M., & Shields, R. K. (2011). Prior heat stress effects fatigue recovery of the elbow flexor muscles. Muscle & Nerve, 44(1), 115-125.
- Kojima, A., Goto, K., Morioka, S., Naito, T., Akema, T., Fujiya, H., … & Yoshioka, T. (2007). Heat stress facilitates the regeneration of injured skeletal muscle in rats. Journal of Orthopaedic Science, 12(1), 74.
- Loenneke, J., Fahs, C., Thiebaud, R., Rossow, L., Abe, T., Ye, X., … & Bemben, M. (2012). The acute muscle swelling effects of blood flow restriction. Acta Physiologica Hungarica, 99(4), 400-410.
- Loenneke, J. P., Kearney, M. L., Thrower, A. D., Collins, S., & Pujol, T. J. (2010). The acute response of practical occlusion in the knee extensors. The Journal of Strength & Conditioning Research, 24(10), 2831-2834.
- Morton, J. P., Croft, L., Bartlett, J. D., MacLaren, D. P., Reilly, T., Evans, L., … & Drust, B. (2009). Reduced carbohydrate availability does not modulate training-induced heat shock protein adaptations but does upregulate oxidative enzyme activity in human skeletal muscle. Journal of Applied Physiology, 106(5), 1513-1521.
- Nosaka, K., Muthalib, M., Lavender, A., & Laursen, P. B. (2007). Attenuation of muscle damage by preconditioning with muscle hyperthermia 1-day prior to eccentric exercise. European Journal of Applied Physiology, 99(2), 183-192.
- Ogonovszky, H., Sasvári, M., Dosek, A., Berkes, I., Kaneko, T., Tahara, S., … & Radák, Z. (2005). The effects of moderate, strenuous, and overtraining on oxidative stress markers and DNA repair in rat liver. Canadian Journal of Applied Physiology, 30(2), 186-195.
- Ohnishi, N., Yamane, M., Uchiyama, N., Shirasawa, S., Kosaka, M., Shiono, H., & Okada, T. (2004). Adaptive changes in muscular performance and circulation by resistance training with regular cold application. Journal of Thermal Biology, 29(7-8), 839-843.
- Peake, J. M., Markworth, J. F., Nosaka, K., Raastad, T., Wadley, G. D., & Coffey, V. G. (2015). Modulating exercise-induced hormesis: does less equal more? Journal of Applied Physiology, 119(3), 172-189.
- Petrella, J. K., Kim, J. S., Mayhew, D. L., Cross, J. M., & Bamman, M. M. (2008). Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. Journal of Applied Physiology, 104(6), 1736-1742.
- Phillips, S. M. (2014). A brief review of critical processes in exercise-induced muscular hypertrophy. Sports Medicine, 44(1), 71-77.
- Radak, Z., Chung, H. Y., Koltai, E., Taylor, A. W., & Goto, S. (2008). Exercise, oxidative stress and hormesis. Ageing Research Reviews, 7(1), 34-42.
- Roberts, L. A., Nosaka, K., Coombes, J. S., & Peake, J. M. (2014). Cold water immersion enhances recovery of submaximal muscle function after resistance exercise. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 307(8), R998-R1008.
- Rubin, B. B., Liauw, S., Tittley, J., Romaschin, A. D., & Walker, P. M. (1992). Prolonged adenine nucleotide resynthesis and reperfusion injury in postischemic skeletal muscle. American Journal of Physiology-Heart and Circulatory Physiology, 262(5), H1538-H1547.
- Saga, N., Katamoto, S., & Naito, H. (2008). Effect of heat preconditioning by microwave hyperthermia on human skeletal muscle after eccentric exercise. Journal of Sports Science & Medicine, 7(1), 176.
- Sandri, M. (2008). Signaling in muscle atrophy and hypertrophy. Physiology, 23(3), 160-170.
- Schoenfeld, B. J., Ogborn, D., & Krieger, J. W. (2017). Dose-response relationship between weekly resistance training volume and increases in muscle mass: A systematic review and meta-analysis. Journal of Sports Sciences, 35(11), 1073-1082.
- Schoenfeld, B. J., Peterson, M. D., Ogborn, D., Contreras, B., & Sonmez, G. T. (2015). Effects of low-vs. high-load resistance training on muscle strength and hypertrophy in well-trained men. The Journal of Strength & Conditioning Research, 29(10), 2954-2963.
- Sekins, K. M., Lehmann, J. F., Esselman, P., Dundore, D., Emery, A. F., & Nelp, W. B. (1984). Local muscle blood flow and temperature responses to 915MHz diathermy as simultaneously measured and numerically predicted. Archives of Physical Medicine and Rehabilitation, 65(1), 1-7.
- Selye, H. (1951). The general-adaptation-syndrome. Annual Review of Medicine, 2(1), 327-342.
- Shima, Y., Kitaoka, K., Yoshiki, Y., Maruhashi, Y., Tsuyama, T., & Tomita, K. (2008). Effect of heat shock preconditioning on ROS scavenging activity in rat skeletal muscle after downhill running. The Journal of Physiological Sciences, 58(5), 341-348.
- Takarada, Y., Nakamura, Y., Aruga, S., Onda, T., Miyazaki, S., & Ishii, N. (2000). Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. Journal of Applied Physiology, 88(1), 61-65.
- Trappe, T. A., White, F., Lambert, C. P., Cesar, D., Hellerstein, M., & Evans, W. J. (2002). Effect of ibuprofen and acetaminophen on postexercise muscle protein synthesis. American Journal of Physiology-Endocrinology and Metabolism, 282(3), E551-E556.
- Wackerhage, H., Schoenfeld, B. J., Hamilton, D. L., Lehti, M., & Hulmi, J. J. (2018). Stimuli and sensors that initiate skeletal muscle hypertrophy following resistance exercise. Journal of Applied Physiology.
- Wernbom, M., Augustsson, J., & Raastad, T. (2008). Ischemic strength training: a low‐load alternative to heavy resistance exercise? Scandinavian Journal of Medicine & Science in Sports, 18(4), 401-416.
- Wernbom, M., Apro, W., Paulsen, G., Nilsen, T. S., Blomstrand, E., & Raastad, T. (2013). Acute low-load resistance exercise with and without blood flow restriction increased protein signalling and number of satellite cells in human skeletal muscle. European Journal of Applied Physiology, 113(12), 2953-2965.
- Wojtaszewski, J. F., MacDonald, C., Nielsen, J. N., Hellsten, Y., Hardie, D. G., Kemp, B. E., … & Richter, E. A. (2003). Regulation of 5′ AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism, 284(4), E813-E822.
- Wyper, D. J., & McNiven, D. R. (1976). The effect of microwave therapy upon muscle blood flow in man. British Journal of Sports Medicine, 10(1), 19-21.
- Yamane, M., Teruya, H., Nakano, M., Ogai, R., Ohnishi, N., & Kosaka, M. (2006). Post-exercise leg and forearm flexor muscle cooling in humans attenuates endurance and resistance training effects on muscle performance and on circulatory adaptation. European Journal of Applied Physiology, 96(5), 572-580.
- Yeo, W. K., McGee, S. L., Carey, A. L., Paton, C. D., Garnham, A. P., Hargreaves, M., & Hawley, J. A. (2010). Acute signalling responses to intense endurance training commenced with low or normal muscle glycogen. Experimental Physiology, 95(2), 351-358.
- Yeo, W. K., Paton, C. D., Garnham, A. P., Burke, L. M., Carey, A. L., & Hawley, J. A. (2008). Skeletal muscle adaptation and performance responses to once a day versus twice every second day endurance training regimens. Journal of Applied Physiology, 105(5), 1462-1470.
From being a mediocre athlete, to professional powerlifter and strength coach, and now to researcher and writer, Charlie combines education and experience in the effort to help Bridge the Gap Between Science and Application. Charlie performs double duty by being the Content Manager for The Muscle PhD as well as the Director of Human Performance at the Applied Science and Performance Institute in Tampa, FL. To appease the nerds, Charlie is a PhD candidate in Human Performance with a master’s degree in Kinesiology and a bachelor’s degree in Exercise Science. For more alphabet soup, Charlie is also a Certified Strength and Conditioning Specialist (CSCS), an ACSM-certified Exercise Physiologist (ACSM-EP), and a USA Weightlifting-certified performance coach (USAW).




